Department of Chemistry and Biochemistry
Dejean Research Group
Areas of Research
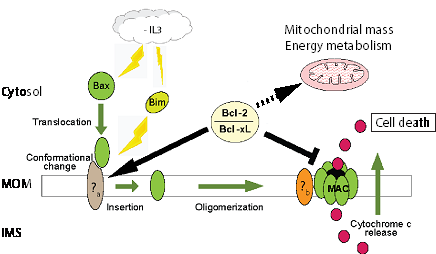
Apoptosis is a cell death program essential to tissue homeostasis, and implicated in a variety of diseases such as cancer and degenerative diseases. Mitochondria play a pivotal role during this process by releasing cytochrome c through the Mitochondrial Apoptosis-induced Channel (MAC). This event is essentially regulated through specific interactions between proteins of the Bcl-2 family that include pro-apoptotic (e.g. Bax), sentinel (e.g. Bim), and anti-apoptotic (e.g. Bcl-2 and Bcl-xL) members. Despite an escalating amount of information on how pro-apoptotic and sentinel proteins act at the mitochondria, the mechanisms by which anti-apoptotic Bcl-2 family proteins inhibit apoptosis remain ill-defined. The Bcl-2 family proteins localize to the mitochondrial outer membrane during the early stages of the apoptotic response. Bax is sequentially translocated to mitochondria, pre-activated through a change of conformation, and then inserted into the outer membrane in response to an apoptotic stimulus. Bax pre-activation leads to its oligomerization, and our group has shown that Bax oligomers are an integral part of MAC. Other proteins of the outer membrane (e.g. Tom22) interact with Bax during this process. Surprisingly, we recently observed that Bax translocation to mitochondria increased in response to an overexpression of the anti-apoptotic Bcl-2 without triggering MAC function. This suggests MAC precursors are accumulated at the mitochondria during Bcl-2/xL up-regulation. Recent studies also found anti-apoptotic Bcl-2 family proteins may be involved in the regulation of mitochondrial mass and energy metabolism. Our work hypothesis is then that the amount of MAC precursors, mitochondrial mass and energy metabolism are positively regulated by Bcl-2/xL levels and ability to bind Bax. This hypothesis is currently tested through experiments of membrane proteins standard biochemistry, mass spectroscopy, molecular biology, and techniques of energetic metabolism studies such as oxygraphy. Completion of this work should provide essential information on the effects of Bcl-2/xL expression levels on the regulation of mitochondrial mass and cell death. We also hope that these studies will identify new potential therapeutic targets, e.g. Bax-interacting proteins as MAC precursors, which could revive the latent apoptotic processes in cancer cells or inhibit it in degenerative diseases.
Recent Publications
- Kinnally, K.W.; Peixoto, P.M.; Ryu, S.; Dejean, L.M. Is mPTP the gatekeeper for necrosis, apoptosis, or both?. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813 (4), 616-622.
- Teijido, O.; Dejean, L. Upregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett. 2010, 584, 3305-3310.
- Dejean, L.M.; Ryu, S.Y.; Peixoto, P.M.; Teijido, O.; Kinnally, K.W. MAC and Bcl-2 family proteins conspire in a deadly plot. Biochim. Biophys. Acta bioenergetics 2010, 1797, 1231-1238.
- Camara, Y.; Mampel, T.; Armengol, J.; Villarroya, F.; Dejean, L. UCP3 expression in liver modulates gene expression and oxidative metabolism in response to fatty acids, and sensitizes mitochondria to permeability transition. Cell. Physiol. Biochem. 2009, 24, 243-252.
- Martinez-Caballero, S.; Dejean, L.M.; Kinnally, M.S.; Oh, K.J.; Mannella, C.A.; Kinnally, K.W. Assembly of the mitochondrial apoptosis-induced channel, MAC. J.Biol.Chem. 2009, 284, 12235-12245.
Recent Presentations
- Teijido Hermida, O.; Tengarai Ganesan, Y.; Renault, T.; Antonsson, B.; Manon, S.; Dejean, L. Interaction between Bcl-2, Bcl-xL, and Bax at the mitochondria: keep your friends close but your enemies closer. Biophysical Society 55th Meeting, Baltimore MD, 2011.
- Teijido Hermida, O.; Antonsson, B.; Dejean, L. Interaction between Bcl-2 and Bax at the mitochondria: keep your friends close but your enemies closer. Gordon Research Conferences on Cell Death, Newport RI, 2010.
- Teijido Hermida, O.; Dejean, L. Upregulation leads Bcl-2 to behave as a mitochondrial decoy receptor for Bax. Biophysical Society 54th Meeting, San Francisco CA, 2010.
- Teijido Hermida, O.; Dejean, L. The product of the proto-oncogene Bcl-2 inhibits Bax insertion during intrinsic apoptosis. Apoptosis and Cancer: The Bcl-2 family proteins meeting, Dartmouth NH, 2009.
- Soprani, A.; Teijido Hermida, O.; Antonsson, B.; Dejean, L. Design of an ELISA protocol for a rapid quantification of activated Bax during apoptosis. Biophysical Society 53rd Meeting, Boston MA, 2009.